Chemical Properties of Tungsten(VI) Chloride
Tungsten(VI) chloride also known as tungsten hexachloride, is a chemical compound with the formula WCl6. As one of the key compounds in tungsten chemistry, this substance is recognized for its high reactivity and versatility in various applications. In this article, we will delve into the intricate details of tungsten(VI) chloride, including its chemical properties, synthesis, industrial uses, and safety precautions. This guide aims to provide an in-depth understanding of WCl6, making it a valuable resource for professionals and researchers in the field of inorganic chemistry.
Chemical Properties of Tungsten(VI) Chloride
Tungsten(VI) chloride is a dark blue, crystalline solid that exists in two polymorphic forms: monoclinic and orthorhombic. The compound is highly reactive, particularly with moisture, making it hygroscopic. When exposed to air, WCl6 readily hydrolyzes to form tungsten oxychloride (WOCl4) and hydrogen chloride (HCl). Its high reactivity is attributed to the presence of the tungsten atom in the +6 oxidation state, which makes the compound a powerful oxidizing agent.
Molecular Structure
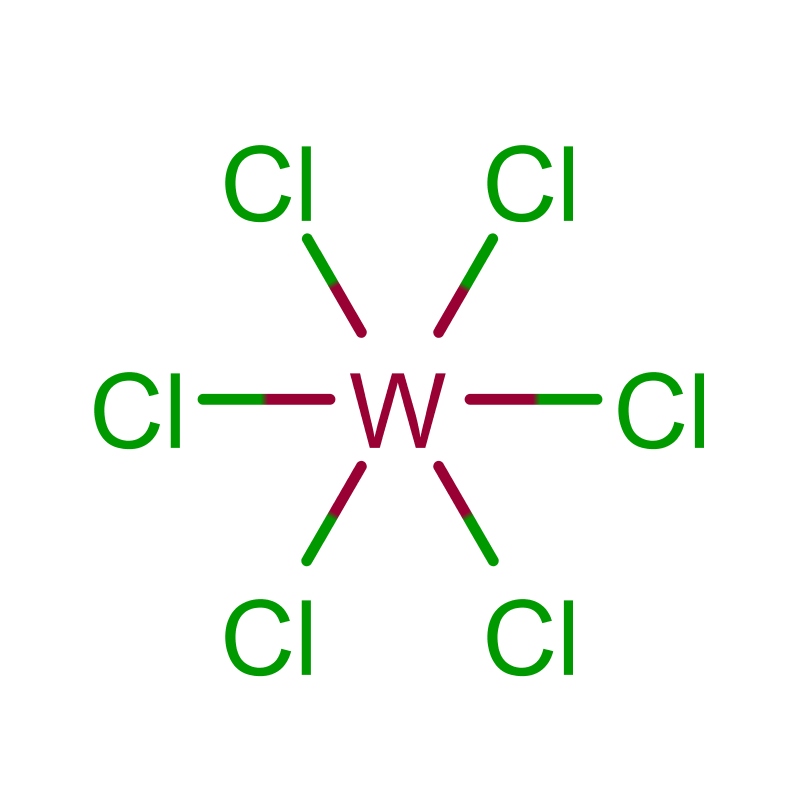
The molecular structure of WCl6 is octahedral, with six chlorine atoms symmetrically surrounding the central tungsten atom. This configuration leads to a highly symmetric molecule, which is characteristic of many metal halides. The W-Cl bond lengths in the molecule are relatively short, contributing to the stability of the compound in its solid state.
Physical Properties
Molecular Weight: 396.56 g/mol
Melting Point: 275°C
Boiling Point: 346°C (decomposes)
Density: 3.79 g/cm³
Appearance: Dark blue crystalline solid
Synthesis of Tungsten(VI) Chloride
The synthesis of tungsten(VI) chloride is typically achieved through the direct chlorination of tungsten metal or tungsten dioxide (WO2) at elevated temperatures. The reaction is carried out in a controlled environment to prevent the formation of unwanted by-products.
Direct Chlorination of Tungsten Metal
The most common method of producing WCl6 involves reacting tungsten metal with chlorine gas at temperatures ranging from 250°C to 350°C. The reaction proceeds as follows:
W (s) + 3 Cl2 (g) → WCl6 (s)
This reaction is highly exothermic and must be carefully controlled to avoid excessive temperatures that could lead to the sublimation of WCl6.
Chlorination of Tungsten Dioxide
Alternatively, tungsten(VI) chloride can be synthesized by chlorinating tungsten dioxide. This method is often used when tungsten metal is not readily available. The reaction is conducted at temperatures around 300°C:
WO2 (s) + 3 Cl2 (g) → WCl6 (s) + O2 (g)
This process also requires precise control to ensure the complete conversion of tungsten dioxide to WCl6.
Conclusion
Tungsten(VI) chloride is a versatile and essential compound in the field of inorganic chemistry, with applications ranging from catalysis to material science. Its unique chemical properties, coupled with its ability to serve as a precursor for other valuable tungsten compounds, make it a critical component in various industrial processes. Understanding the properties, synthesis, and safe handling of WCl6 is crucial for professionals working with this compound.
For more details about Agrochemical and Pharmaceutical intermediates, please contact us.

